Introduction: Although BTK inhibitors (BTKi) are effective therapeutics in the treatment of B cell malignancies, emerging BTK resistance mutations in chronic lymphocytic leukemia (CLL), as well as potential growth-promoting kinase-independent scaffolding function of BTK, present a need for improved or new approaches. Additionally, preclinical and clinical data in non-Hodgkin's lymphoma (NHL) suggest that drugs modulating cereblon may synergize with BTKi to provide a therapeutic effect. NX-2127 is an oral, first-in-class, dual-function small molecule degrader that combines BTK degradation with the immunomodulatory activity of an Ikaros and Aiolos degrader. Preliminary safety of NX-2127 in patients across B cell malignancies and efficacy in patients with CLL have been presented previously [Mato et al. 2022; Danilov et al. 2023]. Here we report further safety and efficacy follow-up in patients with CLL and efficacy data in patients with NHL enrolled to date.
Methods: NX-2127-001 (NCT04830137) is a first-in-human, multicenter, open-label, dose-escalation (Phase 1a) and cohort-expansion (Phase 1b) trial evaluating the safety and preliminary efficacy of NX-2127 in adults with relapsed/refractory B cell malignancies, including CLL, diffuse large B cell lymphoma (DLBCL), follicular lymphoma (FL), mantle cell lymphoma (MCL), marginal zone lymphoma (MZL), and Waldenstrom's macroglobulinemia (WM). NX-2127 is administered orally once daily in 28-day cycles.
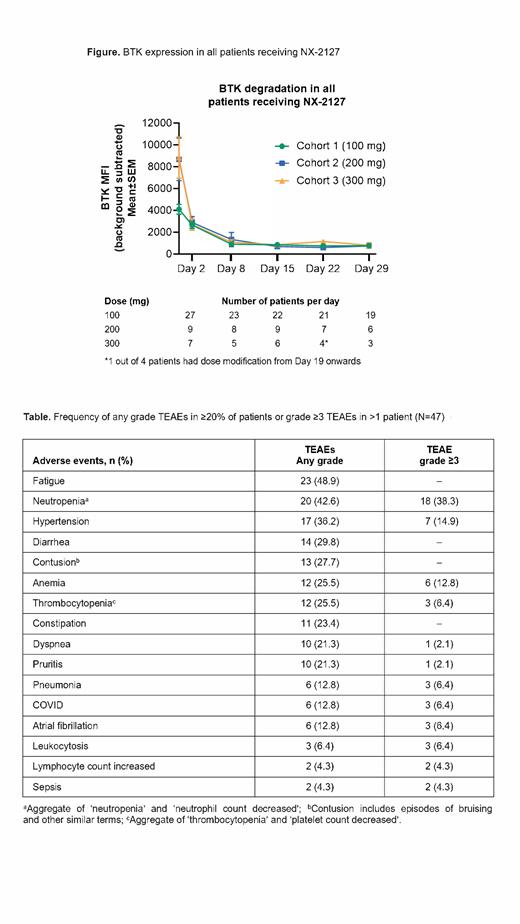
Results: As of 9 June 2023, 47 patients were enrolled and treated with NX-2127 at once-daily doses of 100 mg (n=28), 200 mg (n=10), and 300 mg (n=9). Patients were predominantly male (66%), with a median age of 74 (range 50-92) years. Twenty-nine patients were treated for CLL/small lymphocytic lymphoma, 5 DLBCL (2 GCB [Germinal-center B-cell-like], 3 non-GCB), 5 MCL, 3 MZL, 3 WM, and 2 FL. Patients enrolled were heavily pretreated with median prior lines of therapy of 4 (range 2-10) in NHL and 5 (range 2-11) in CLL. Prior treatments in patients with CLL comprised: BTKi 100% (including covalent [cBTKi] and non-covalent [ncBTKi] inhibitors); BCL2 inhibitor 76%, with a large proportion of CLL patients exhibiting BTKi resistance mutations at baseline. Prior treatments in patients with NHL included: BTKi 72% (cBTKi and ncBTKi); bispecific antibody 1/18; bispecific antibody and CAR-T 1/18. There were two reported dose-limiting toxicities: one previously reported cognitive disturbance in a patient with CLL treated at 300 mg; and neutropenia in a patient with MZL treated at 300 mg. The most common any grade treatment-emergent adverse events (TEAEs) were fatigue (48.9%), neutropenia (42.6%) and hypertension (36.2%, see Table). The most common grade ≥3 TEAEs were neutropenia (38.3%), hypertension (14.9%) and anemia (12.8%). Contusion was reported in 27.7% of patients (all below grade 3), atrial fibrillation in 12.8% of patients (6.4% grade ≥3). Most common reasons for treatment discontinuation were progressive disease (PD, 25.5%) and AE (21.3%). Median follow-up for the study was 9.5 (range 0.1-24.3) months. NX-2127 exhibited dose-dependent pharmacokinetics (PK) with a mean half-life of 2-4 days across cohorts. Rapid, robust and sustained BTK degradation was observed in all patients, regardless of their absolute BTK starting level, tumor type, or dose level of NX-2127 (Figure). In addition, degradation of the cereblon neo-substrate Ikaros was observed. Among the efficacy evaluable patients with CLL, there were 9 PRs/PR with rebound lymphocytosis; additionally, 11 patients had SD at the time of data cut-off and 4 had PD. Two patients with WM were treated and efficacy evaluable (1 SD, 1 PD). Among the efficacy evaluable patients with NHL, there were 2 CRs and 1 PR; additionally, 3 patients had SD, and 5 had PD. Two CRs are ongoing with 9.2 and 11.8 months of duration.
Conclusion: This first-in-human study of NX-2127 is actively enrolling and dose-expansion cohorts in DLBCL and MCL have been initiated at the 300 mg daily dose. Findings include dose-dependent PK accompanied by degradation of BTK and Ikaros. Encouraging and persistent responses were observed in heavily pretreated patients with relapsed/refractory CLL and NHL with a manageable safety profile. These data support the treatment concept of combining immunomodulatory activity and BTK degradation in a single molecule and support further development of NX-2127 in B cell malignancies.
OffLabel Disclosure:
Danilov:MEI: Consultancy, Research Funding; Lilly Oncology: Consultancy, Research Funding; Cyclacel: Research Funding; Nurix: Consultancy, Research Funding; Bayer: Research Funding; Abbvie: Consultancy, Research Funding; Beigene: Consultancy, Research Funding; Janssen: Consultancy; Astra Zeneca: Consultancy, Research Funding; Bristol Meyers Squibb: Consultancy, Research Funding; Genentech: Consultancy; GenMab: Consultancy, Research Funding; Merck: Consultancy. Patel:Adaptive Biotechnelogies, AstraZeneca, Bristol Myers Squibb, CRISPR Therapeutics, Curis, Inc, Epizyme, Fate Therapeutics, Genentech, Inc. / F. Hoffmann-La Roche Ltd, Kite, Loxo Oncology, MEI Pharma, Merck, Nurix, Pharmacyclics/Janssen, Sunesis Pharmaceuti: Research Funding; Abbvie, ADC Therapeutics, AstraZeneca, BeiGene, Bristol Myers Squibb, Caribou Biosciences, Epizyme, Genentech, Inc. / F. Hoffmann-La Roche Ltd, Kite, Loxo Oncology, MEI Pharma, Merck, Morphosys, Nurix, Pharmacyclics/Janssen, Sana Biotechnology, TG Therape: Consultancy; AstraZeneca, Bristol Myers Squibb, Kite, TG Therapeutics: Speakers Bureau. Wierda:Janssens Biotech: Research Funding; National Comprehensive Cancer Network: Other: Nonrelevant Financial Relationship/Chair, CLL). Supported by the NIH/NCI under award number P30 CA016672 and used MDACC Cancer Center Support Grant (CCSG) shared resources; Accutar Biotechnology: Research Funding; Juno Therapeutics: Research Funding; Pharmacyclics LLC: Research Funding; NIH P30 CA016672/MDACC Cancer Center Support Grant: Research Funding; Numab THerapeutics: Research Funding; Nurix THerapeutics: Research Funding; Loxo Oncology, Inc./Lilly: Research Funding; AstraZeneca/Acerta Pharma: Consultancy, Research Funding; Bristol Myers Squibb (Juno & Celgene): Consultancy, Research Funding; Gilead Sciences: Research Funding; GlaxoSmithKline: Research Funding; AbbVie: Consultancy, Research Funding; Genentech: Research Funding; Janssens Biotech Inc: Research Funding; GSK/Novartis: Research Funding; Cyclacel: Consultancy, Research Funding; Oncternal Therapeutics, Inc.: Research Funding; Sunesis: Research Funding; Miragen: Research Funding; KITE Pharma: Research Funding. Patel:Olema Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; ION Pharmaceuticals: Other: Leadership; Janssen Oncology: Honoraria. Flinn:Servier Pharma: Consultancy; Secura Bio: Consultancy; Novartis: Consultancy; Myeloid Therapeutics: Consultancy; Kite: Consultancy; Innocare Pharma: Consultancy; Hutchinson MediPharma: Consultancy; Genmab: Consultancy; Genentech: Consultancy; Century Therapeutics: Consultancy; BeiGene: Consultancy; AbbVie: Consultancy; TG Therapeutics: Consultancy; Vincerx Pharma: Consultancy. Ai:Biomerieux: Honoraria; Kyowa Kirin: Honoraria; Secura Bio: Membership on an entity's Board of Directors or advisory committees. Thompson:AstraZeneca: Consultancy; Genentech: Research Funding; Abbvie: Research Funding; Intellisphere LLC: Honoraria; AstraZeneca: Research Funding; Genmab: Research Funding; MJH Life Sciences: Honoraria; Loxo Oncology at Lilly: Consultancy; Dava Oncology: Other: Travel ; Brazilian Association of Hematology, Hemotherapy and Cellular Therapy (ABHH): Honoraria; Curio Science: Honoraria; VJHemOnc: Honoraria; Massachusetts Medical Society: Honoraria; Janssen: Consultancy; Nurix Therapeutics: Research Funding; Beigene: Research Funding. Wang:Genentech: Consultancy, Research Funding; MD Education: Honoraria; MJH Life Sciences: Honoraria; WebMD: Honoraria; Scripps: Honoraria; Studio ER Congressi: Honoraria; Nurix: Honoraria; OncLive: Honoraria; Loxo Oncology: Consultancy, Research Funding; Moffit Cancer Center: Honoraria; Anticancer Association: Honoraria; Molecular Templates: Research Funding; IDEOlogy Health: Honoraria; Vincerx: Research Funding; i3Health: Honoraria; Medscape: Honoraria; Eastern Virginia Medical School: Honoraria; Dava Oncology: Honoraria, Other: Travel; Celgene: Other: Travel, Research Funding; BGICS: Honoraria; Meeting Minds Experts: Honoraria; Genmab: Honoraria, Research Funding; Clinical Care Options: Honoraria; Epizyme: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria, Other: Travel, Research Funding; CAHON: Honoraria; Bantam Pharmaceutical: Honoraria; VelosBio: Consultancy, Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; Pepromene Bio: Consultancy; Parexel: Consultancy; Oncternal: Consultancy, Research Funding; Milken Institute: Consultancy; Miltenyi Biomedicine: Consultancy; Merck: Consultancy, Honoraria; NIH: Honoraria; Be Biopharma: Consultancy; BeiGene: Consultancy, Honoraria, Research Funding; Oncology Specialty Group: Honoraria; Physicians Education Resources (PER): Honoraria, Other: Travel; Practice Point Communications (PPC): Honoraria; DTRM Biopharma (Cayman) Limited: Consultancy; Deciphera: Consultancy; Bristol Myers Squibb: Consultancy, Honoraria; BioInvent: Consultancy, Honoraria, Research Funding; Eli Lilly and Company: Consultancy, Research Funding; Leukemia & Lymphoma Society: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; InnoCare: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Juno Therapeutics: Research Funding; OMI: Honoraria; Pharmacyclics: Honoraria; Physicians Education Resources: Honoraria; Practice Point Communications: Honoraria; CSTone: Consultancy; Amphista Therapeutics Limited: Consultancy; ADC Therapeutics America: Consultancy; Acerta Pharma: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria; Hebei Cancer Prevention Federation: Honoraria; Imedex: Honoraria; TS Oncology: Honoraria; Mumbai Hematology Group: Honoraria. Sun:Genmab: Research Funding. Stephens:AbbVie: Consultancy; AstraZeneca: Consultancy, Research Funding; BeiGene: Consultancy; Bristol-Myers Squibb: Consultancy; Celgene: Consultancy; Genentech: Consultancy; Janssen: Consultancy; Lilly: Consultancy; Novartis: Research Funding. Thirman:Syndax: Research Funding; AbbVie: Honoraria; AbbVie: Research Funding; Merck: Research Funding; Nurix: Research Funding. Gessner:Nurix Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company. Wolff:Nurix Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company. Schwab:Nurix Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company. Tan:Nurix Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company. Chan:Nurix Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company. Meredith:Nurix Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company. Wiestner:Genmab: Research Funding; Acerta: Research Funding; Pharmacyclics: Research Funding; Merck: Research Funding; Nurix: Research Funding; Secura Bio: Research Funding.
NX-2127 is an oral, first-in-class, dual-function small molecule degrader that combines BTK degradation with the immunomodulatory activity of an Ikaros and Aiolos degrader.